 Catalog Products Catalog Products |
|
| Chelators | | | Synthesis Accessories | | | 合成试剂 | | | 合成树脂 | |
|
 |
| Product | Fmoc - TOAC - OH
| | CAS | | | Size | 5 mg | | Catalog # | 62658 | | US$ | 242 | | Hits | |
| Description |
Fmoc-TOAC–OH is used for the synthesis of TOAC-peptides.
TOAC (2,2,6,6-tetramethylpiperidine-1oxyl-4-amino-4-carboxylic acid) is a nitroxide spin-labeled, achiral Cα-tetrasubstituted unnatural amino acid and an excellent tool in material science and biochemistry. Recently, TOAC has been shown to be an effective β-turn and 310/α-helix promoter in peptides and an excellent and relatively rigid electron paramagnetic resonance (EPR) probe and fluorescence quencher.
Publications have reported the use of Fmoc-TOAC-OH in the synthesis of internally containing spin probe peptide sequence, which was previously not possible with BOC protected TOAC. The incorporation of this amino acid has also been reported in the synthesis of a biologically active hormone.
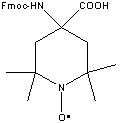
|
| Storage |
4°C |
| References |
Rassat, A. and P. Rey. Bull. Soc. Chim. Fr. 3, 815 (1967). Barbosa, SR. et al. FEBS Lett. 446, 45 (1999). Marchetto, R. et al. J. Am. Chem. Soc. 115, 11042 (1993). Toniolo, C., Crisma, M & Formaggio, F. Biopolymers (Peptide Sci.) 47, 153 (1998); Bui,T.T.T., Formaggio, F., et al. J. Chem. Soc., Perkin Trans. 2, 1043 (2000); Polese, A., Anderson, D.J., et al. J. Am. Chem. Soc. 121, 11071 (1999); Martin, L., Ivancich, A., et al. J. Pept. Res. 58, 424 (2001). Rusiecki, VK. & SA. Warne Bioorg. Med. Chem. Lett., 3, 707 (1993). Tatsu, Y. et al. In “Dynamic Studies in Biology: Phototriggers, Photoswitches and Caged. Wiley-VCH (2005); Marchetto, R. et al. J. Am. Chem. Soc. 115, 11042 (1993). |
| Molecular Weight |
437.51 |
| Molecular Formula |
C25H29N2O5 |
|
|