 Catalog Products Catalog Products |
|
| β-Amino Acids | | | Aliphatic Amino Acids | | | Analogs of Alanine, Glycine, Valine, and Leucine | | | Analogs of Arginine and Lysine | | | Analogs of Aspartic and Glutamic Acids | | | Analogs of Benzoic Acid | | | Analogs of Cysteine and Methionine | | | Analogs of Phenylalanine and Tyrosine | | | Analogs of Proline | | | Analogs of Serine, Threonine, and Statine | | | Analogs of Tryptophan | | | Aromatic Amino Acids | | | Azide and Alkyne containing Amino Acids | | | Building Blocks for Stapled Peptides | | | Depsipeptides | | | Dye-labeled Amino Acids | | | Heterocyclic Amino Acids | | | N-α-Methyl Amino Acids | | | PEGylated Amino Acids | | | PTM Building Blocks | |
|
 |
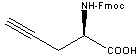
| Product | Fmoc - D - propargylglycine
Fmoc - D - Pra - OH | | CAS | Fmoc - D - Pra - OH | | Size | 1 g | | Catalog # | 26265-F1 | | US$ | 181 | | Hits | |
| Description |
An unusual amino acid used in Solid Phase Peptide Synthesis (SPPS) as: A) a precursor to the tritium-induced Norvaline peptides1, B) in the synthesis of branched peptides using a palladium-catalyzed coupling reaction2, and C) as a reagent in the dual-catalyst Sonogashira coupling reaction.3
|
| Detailed Information |
 Material Safety Data Sheets (MSDS) Material Safety Data Sheets (MSDS)
 Flyer Flyer |
| Storage |
4°C |
| References |
1. Eberle, A. N.; Zeller, A. Tritiation of Peptides to High Specific Radioactivity. Part 1. Synthesis and Biological Properties of [13-(3H4)Norvaline]-α-MSH and of [2,23-Bis((3H2)tyrosine)]ACTH(1-24). Helvetica Chim. Acta. 1985 (68) 1880-1892.
2. Crisp, G. T.; Robertson, T. A. Palladium-catalyzed coupling of a propargylglycinededrivative. Tetrahedron. 1992 (48) 3239-3250.
3. Sonogashira, K.; Tohda, Y.; Haghiara, N. A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes, and bromopyridines.Tetrahedron Lett.,1975 (16) 4467-4470. |
| Molecular Weight |
335.4 |
| Molecular Formula |
C20H17NO4 |
|
|