 Catalog Products Catalog Products |
|
| β-Amino Acids | | | Aliphatic Amino Acids | | | Analogs of Alanine, Glycine, Valine, and Leucine | | | Analogs of Arginine and Lysine | | | Analogs of Aspartic and Glutamic Acids | | | Analogs of Benzoic Acid | | | Analogs of Cysteine and Methionine | | | Analogs of Phenylalanine and Tyrosine | | | Analogs of Proline | | | Analogs of Serine, Threonine, and Statine | | | Analogs of Tryptophan | | | Aromatic Amino Acids | | | Azide and Alkyne containing Amino Acids | | | Building Blocks for Stapled Peptides | | | Depsipeptides | | | Dye-labeled Amino Acids | | | Heterocyclic Amino Acids | | | N-α-Methyl Amino Acids | | | PEGylated Amino Acids | | | PTM Building Blocks | |
|
 |
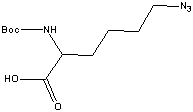
| Product | Boc - Lys(N3) - OHBoc - azidolysine; Boc - lys(azide);
Boc - lysine azide | | CAS | Boc - lysine azide | | Size | 250 mg | | Catalog # | 53100-B025 | | US$ | 154 | | Hits | |
| Description |
An unusual amino acid used in Solid Phase Peptide Synthesis (SPPS) for the synthesis of side-chain modified peptides and proteins.1 The side-chain azido (N3) group is stable in trifluoroacetic acid (TFA) or piperidine (Pip), two reagents commonly used in SPPS. The azido group can be readily converted to an amine on the solid phase or in solution via the Staudinger reduction.2
|
| Detailed Information |
 Catalog Catalog
 Flyer Flyer |
| Storage |
4°C |
| References |
1. Vallee, M. R. J.; Majkut, P.; Wilkening, C. W.; Muller, G.; Hackenberger, C. P. R. Staudinger-Phosphonite Reactions for the Chemoselective Transformation of Azido-Containing Peptides and Proteins.Org. Lett. 2011 (13) 5440-5443.
2. Staudinger, H.; Meyer, J. UberneueorganischePhosphorverbindungen III. Phosphinmethylenederivative und Phosphinimine. Helvetica Chim. Acta. 1919 (2) 635-646. |
| Molecular Weight |
272.3 |
| Molecular Formula |
C11H20N4O4 |
|
|